The same proteins of Example 2 were compared using a representation that includes the C-alpha and the geometric centroid of the side chains. The two proteins involved belong to the well-known group of Rossmann-like folds. This structure is characterized by a central beta-sheet in which alhpha-helices connect the strands together. One of these beta-alpha-beta units contains a characteristic glycine-rich phosphate binding loop [Schulz GE, Curr Opin Struct Biol. (1992), 2:61–67].
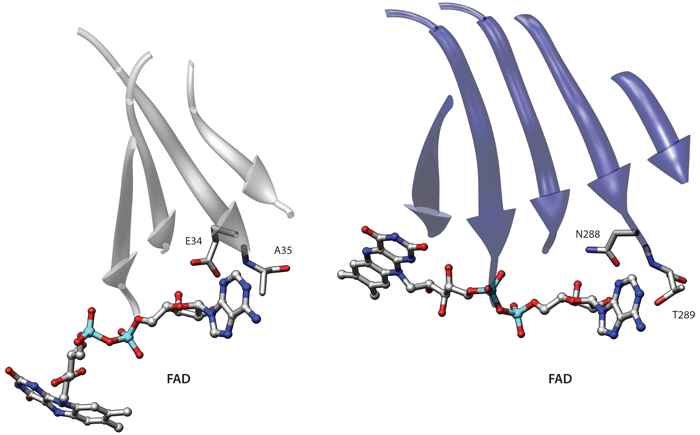
Interestingly the beta-sheets of the monoamine oxidase and the electron transfer flavoprotein have a permuted structure. In the first protein the phosphate binding loop occurs in the N-terminal beta-alpha-beta unit of the sheet. Conversely the phosphate binding loop of the electron transfer flavoprotein is located in the second beta-alpha-beta unit. The two beta-sheets also have a different twist. Indeed if one superimposes the two beta-sheets in an optimal way the ligand binding sites end up in opposite positions. The match shown in Example 2 comprises eight residues, captures the overall similarity between these two binding sites and places the phosphate binding elements in similar positions. However in terms of the overall structures the beta-sheets are placed one in front of the other.
If we include side-chain information and re-run the comparison we obtain an interesting albeit much smaller similarity. Indeed as shown in the picture in this page these proteins also have an identical adenine binding motif, which was also described by Denessiouk and Johnson [Denessiouk KA, Johnson MS, J Mol Biol (2003), 333(5):1025-43] (the residues comprising the beta-sheets were not used in the comparison). This second alignment is also informative because it highlights a similar adenine recognition site in these proteins. In this alignment the central beta-sheets are closer in space, even though an alignment that simultaneously superimposes both the central sheets and the binding sites is not possible due to the above-mentioned permutation. Therefore these two proteins provide an interesting example of the results that can obtained using different levels of detail in the representation.

